Disulfide bond rearrangement during regioselective oxidation in PhS(O)Ph/CH3SiCl3 mixture for the synthesis of alpha-conotoxin GI.
Ildikó Szabó, Gitta Schlosser, Ferenc Hudecz, Gábor Mezo
文献索引:Biopolymers 88(1) , 20-8, (2007)
全文:HTML全文
摘要
Rearrangement of disulfide bonds during the synthesis of alpha-conotoxin GI using PhS(O)Ph/CH(3)SiCl(3) oxidation procedure was observed. We have demonstrated that the protecting scheme (order of acetamidomethyl (Acm) and (t)Bu protecting groups) of the Cys residues as well as the reaction time influenced the ratio of the native and the mispaired compounds, while the temperature of the reaction mixture had no significant effect. However, in all cases the nonnative derivative was produced in high amount. The structure of the isomers was identified by the combination of enzymatic digestion and mass spectrometry measurements. We conclude that the air oxidation followed by the application of Tl(tfa)(3) for the regioselective formation of disulfide bonds leads up to the appropriate compound in the case of the synthesis of alpha-conotoxin GI, while the oxidation procedure using PhS(O)Ph/CH(3)SiCl(3) system resulted in the nonnative disulfide isomer.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
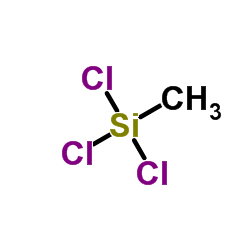 |
甲基三氯硅烷
CAS:75-79-6 |
CH3Cl3Si |
|
Enzyme electrode for the measurement of lactate in undiluted...
1986-06-15 [Clin. Chim. Acta 157(2) , 191-8, (1986)] |
|
Theoretical study of the pyrolysis of methyltrichlorosilane ...
2010-02-18 [J. Phys. Chem. A 114(6) , 2384-92, (2010)] |
|
Theoretical study of the pyrolysis of methyltrichlorosilane ...
2007-03-01 [J. Phys. Chem. A 111(8) , 1475-86, (2007)] |
|
Preparation of highly hydrophobic and lipophobic cellulose f...
2010-04-15 [J. Colloid. Interface Sci. 344(2) , 588-95, (2010)] |
|
Theoretical study of the pyrolysis of methyltrichlorosilane ...
2007-03-01 [J. Phys. Chem. A 111(8) , 1462-74, (2007)] |