European Biophysics Journal
1994-01-01
The conformation of linear gramicidin is sequence dependent. A monolayer and infrared study.
N Van Mau, B Bonnet, A Benayad, F Heitz
文献索引:Eur. Biophys. J. 22(6) , 447-52, (1994)
全文:HTML全文
摘要
A comparative monolayer and infrared study of analogues of gramicidin A containing either tyrosines or naphthylalanines instead of tryptophans indicates that the nature of the aromatic residues influences the favoured conformation of the peptides. Polar residues favour the single stranded IIDL helix while non polar residues favour the double stranded helix. For partly tryptophan to naphthylalanine substituted analogues the positions of the substitutions orientate the favored conformation. The nature of these substitutions may also modify the peptide-lipid interactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
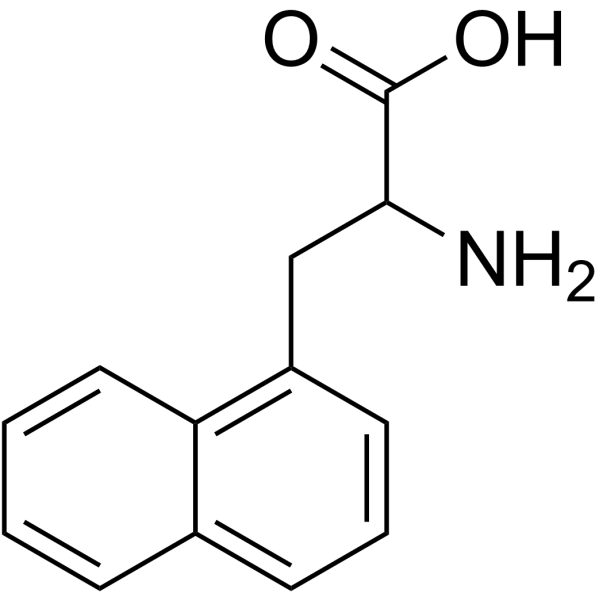 |
3-(1-萘基)丙氨酸
CAS:28095-56-9 |
C13H13NO2 |
相关文献:
更多...
|
Synthesis and characterization of more potent analogues of h...
1998-09-29 [Biochemistry 37(39) , 13507-15, (1998)] |
|
The A14 position of insulin tolerates considerable structura...
1992-10-01 [J. Protein Chem. 11(5) , 571-7, (1992)] |
|
Adding L-3-(2-Naphthyl)alanine to the genetic code of E. col...
2002-03-06 [J. Am. Chem. Soc. 124 , 1836-1837, (2002)] |
|
Conformational analyses of cyclic hexapeptide analogs of som...
1999-04-01 [J. Pept. Sci. 5(4) , 161-75, (1999)] |
|
Synthesis and binding properties of cyclohexapeptide somatos...
1999-03-01 [J. Pept. Sci. 5(3) , 113-30, (1999)] |