| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
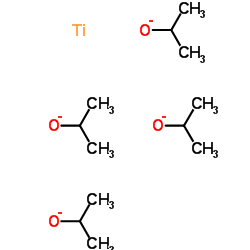 |
四异丙醇钛
CAS:546-68-9 |
|
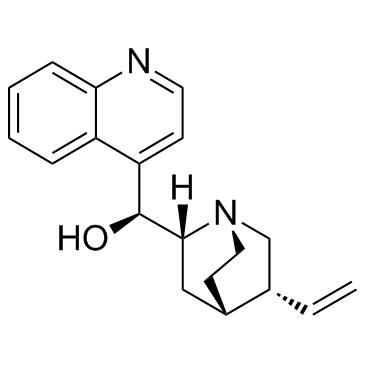 |
辛可宁
CAS:118-10-5 |
|
 |
弱金鸡纳碱硫酸盐二水合物
CAS:5949-16-6 |
|
 |
2,2'-二羟基联苯
CAS:1806-29-7 |