| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
(S)-(-)-4-氧代-2-氮杂环丁烷甲酸苄酯
CAS:72776-05-7 |
|
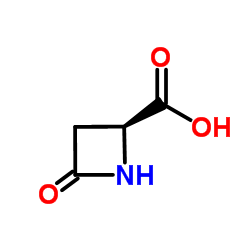 |
(S)-(-)-4-氧代-2-吖丁啶羧酸
CAS:16404-94-7 |