| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
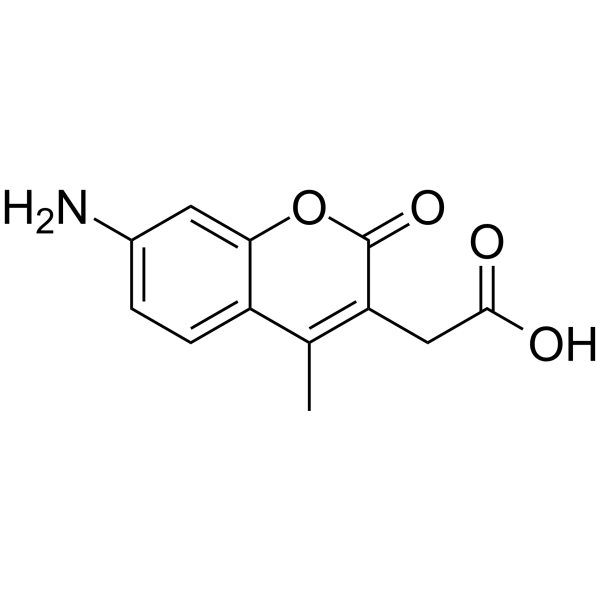 |
7-氨基-4-甲基香豆素-3-乙酸(AMCA)
CAS:106562-32-7 |
|
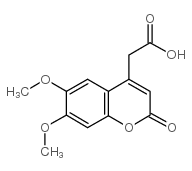 |
6,7-二甲氧基香豆素-4-乙酸
CAS:88404-26-6 |