| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
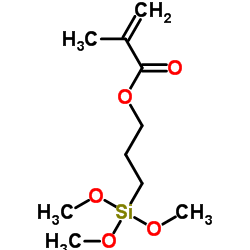 |
3-(甲基丙烯酰氧)丙基三甲氧基硅烷
CAS:2530-85-0 |
|
 |
双酚a
CAS:80-05-7 |
|
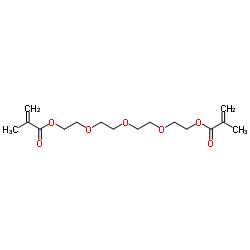 |
四乙二醇二甲基丙烯酸酯
CAS:109-17-1 |