A neolignan-type impurity arising from the peracid oxidation reaction of anethole in the surreptitious synthesis of 4-methoxyamphetamine (PMA).
Dieter Waumans, Bas Hermans, Noël Bruneel, Jan Tytgat
文献索引:Forensic Sci. Int. 143(2-3) , 133-9, (2004)
全文:HTML全文
摘要
The neolignan-type substance 2,4-dimethyl-3,5-bis(4'-methoxyphenyl) tetrahydrofuran is presented as a new forensic marker compound for the peracid oxidation of anethole. It is hypothesized that the formation of a stable intermediary carbocation in the hydrolysis reaction of anethole epoxide is not only responsible for the presence of 1,2-diols (and its esters) and 4-methoxyphenyl-2-propanone (PMP2P) but can also be the cause for the creation of this neolignan impurity due to interaction with anethole itself. Moreover, the applicability of this new forensic marker is demonstrated by its retrieval in clandestinely manufactured 4-methoxyamphetamine (PMA) preparations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
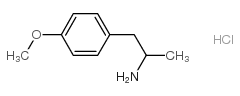 |
盐酸甲氧安非他明
CAS:52740-56-4 |
C10H16ClNO |
|
Studies on distribution and metabolism of para-methoxymetham...
2009-05-02 [Toxicology 259(1-2) , 61-8, (2009)] |
|
The effects of co-administration of 3,4-methylenedioxymetham...
2007-04-25 [Neuroscience 146(1) , 321-9, (2007)] |
|
Effects of 3,4-methylenedioxymethamphetamine (MDMA, 'Ecstasy...
2005-04-11 [Brain Res. 1041(1) , 48-55, (2005)] |
|
Selective electrochemical discrimination between dopamine an...
2010-06-01 [Analyst 135(6) , 1449-55, (2010)] |
|
Repeated administration of the substituted amphetamine p-met...
2006-09-28 [Eur. J. Pharmacol. 546(1-3) , 74-81, (2006)] |