Stereoselectivity in Diels-Alder reactions of diene-substituted N-alkoxycarbonyl-1,2-dihydropyridines.
Grant R Krow, Qiuli Huang, Steven W Szczepanski, Fredrick H Hausheer, Patrick J Carroll
文献索引:J. Org. Chem. 72(9) , 3458-66, (2007)
全文:HTML全文
摘要
Diene substituent effects on the regiochemical and stereochemical outcomes of uncatalyzed Diels-Alder reactions of N-alkoxycarbonyl-1,2-dihydropyridines with both styrene and methyl vinyl ketone (MVK) were studied. Alkyl substitution on the diene in all cases examined resulted in a kinetic preference for 7-endo isomers (7-phenyl 51-96% exo and 7-acetyl 54-96% exo). For both dienophiles, the highest stereoselectivities (>or=89% endo) were observed with 5-methyl or 6-methyl substituents in the dihydropyridine. Theoretical calculations of the energies of gas phase endo and exo transition states at the RHF/3-21G(*) predict that total entropy, DeltaStotal, considerations favor endo cycloadducts for both dienophiles with DHP, while total energy considerations, DeltaEo, favor endo cycloadducts for styrene and exo cycloadducts for MVK. At this level, favored endo-phenyl isomers are correctly predicted for styrene reactions, but the calculation of 7-acetyl exo or endo isomer dominance is diene-substituent-dependent for MVK reactions. The preference for endo addition of MVK to the parent, 5-methyl, and 6-methyl-DHPs was successfully predicted by calculations at the B3LYP/6-31G* theory level.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
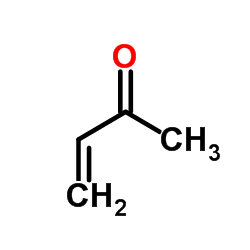 |
丁烯酮
CAS:78-94-4 |
C4H6O |
|
Modulation of mitochondrial glutathione status and cellular ...
2013-05-01 [Biochem. Pharmacol. 85(9) , 1379-88, (2013)] |
|
Methyl vinyl ketone, a toxic ingredient in cigarette smoke e...
2014-01-01 [Chem. Pharm. Bull. 62(8) , 772-8, (2014)] |
|
Quantum mechanical/molecular mechanical modeling finds Diels...
2010-03-10 [J. Am. Chem. Soc. 132(9) , 3097-104, (2010)] |
|
Enantioselective Michael addition of 3-aryl-substituted oxin...
2012-01-01 [Molecules 17(6) , 7523-32, (2012)] |
|
Diabetes increases susceptibility of primary cultures of rat...
2009-11-15 [Toxicol. Appl. Pharmacol. 241(1) , 1-13, (2009)] |