Mutagenesis
2008-07-01
Up-regulation of the glutathione S-transferase system in human liver by polycyclic aromatic hydrocarbons; comparison with rat liver and lung.
Daphnee S Pushparajah, Meera Umachandran, Kathryn E Plant, Nick Plant, Costas Ioannides
文献索引:Mutagenesis 23(4) , 299-308, (2008)
全文:HTML全文
摘要
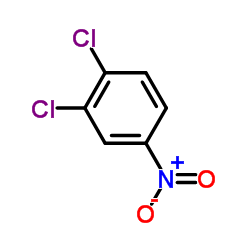
The cytosolic glutathione S-transferases (GSTs) comprise a pivotal enzyme system protecting the cell from electrophilic compounds. It plays a major role in the detoxication of the primary and dihydrodiol epoxides of polycyclic aromatic hydrocarbons (PAHs), so that modulation of this enzyme system by PAHs will impact on their carcinogenic activity. The potential of six structurally diverse PAHs, namely benzo[a]pyrene (B[a]P), fluoranthene, benzo[b]fluoranthene (B[b]F), dibenzo[a,l]pyrene, dibenzo[a,h]anthracene (D[a,h]A) and 1-methhylphenanthrene, to modulate hepatic GST activity was investigated in human precision-cut slices and compared to rat slices, a species frequently used in long-term carcinogenicity studies; changes were monitored at the activity, using three different substrates, protein and mRNA levels. When activity was monitored using the alpha-class selective 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole, B[b]F was the only PAH that caused an increase in activity, which was accompanied by a rise in the Ya immunoreacting band. In rat slices, in addition to B[b]F, B[a]P and D[a,h]A also enhanced activity, being paralleled with increased levels of the Ya immunoreacting band. In the rat, all PAHs elevated mRNA levels. In both human and rat liver slices, only B[b]F enhanced activity when 1-chloro-2,4-dinitrobenzene (CDNB) served as substrate. To investigate tissue differences, similar studies were undertaken in precision-cut rat lung slices, incubated with PAHs under identical conditions, using CDNB, as this was the only substrate for which activity could be detected; none of the PAHs studied stimulated activity. It is concluded that some PAHs have the potential to induce GST activity in human liver tissue and that species and tissue differences exist in the induction of this enzyme system in the rat. However, the extent of induction of GST activity is very modest compared with the effect these compounds have on CYP1 expression, the family responsible for their bioactivation, and it is unlikely to compensate for the enhanced production of reactive intermediates.
相关化合物