| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
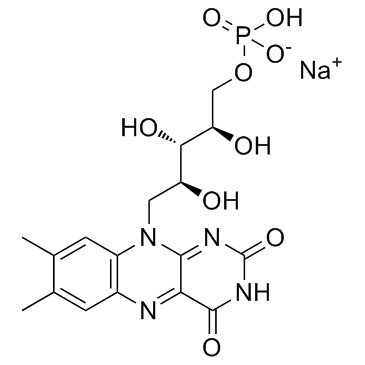 |
核黄素磷酸钠
CAS:130-40-5 |
|
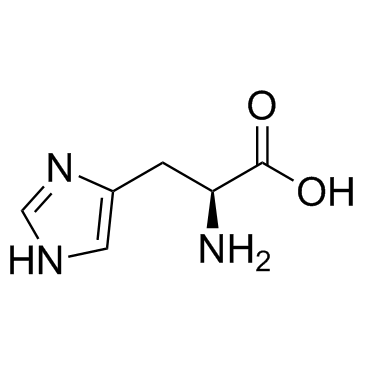 |
L-组氨酸
CAS:71-00-1 |
|
 |
铁氧化还原蛋白-NADP + 还原酶 来源于菠菜
CAS:9029-33-8 |
|
 |
细胞色素P450还原酶
CAS:9039-06-9 |