Highly enantioselective mukaiyama aldol reaction of alpha,alpha-dichloro ketene silyl acetal: an efficient synthesis of a key intermediate for diltiazem.
Ritsuo Imashiro, Tooru Kuroda
文献索引:J. Org. Chem. 68(3) , 974-9, (2003)
全文:HTML全文
摘要
An efficient synthesis of methyl (2R,3S)-3-(4-methoxyphenyl)glycidate (-)-2, a key intermediate for diltiazem (1), has been developed on the basis of the highly enantioselective Mukaiyama aldol reaction of p-anisaldehyde (4a) with alpha,alpha-dichloro ketene silyl acetal 5. Thus, the reaction using a stoichiometric amount of chiral oxazaborolidinone catalyst 12a proceeded to excellent yield (83%) and high enantioselectivity (96% ee), together with the chiral ligand 13a in nearly quantitative recovery. The reaction using a substoichiometric amount of 12e (20 mol %) also proceeded to excellent yield (88%), with somewhat lower enantioselectivity (77% ee). The aldol product 3a thus obtained was easily converted to (-)-2 in excellent yield (80%) and high optical purity (>99% ee). The highly enantioselective Mukaiyama aldol reaction with 5 catalyzed by 12a proved to be applicable to various aldehydes. An efficient preparation of 5 from inexpensive starting materials was also described.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
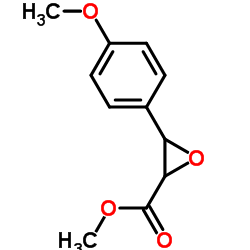 |
3-(4-甲氧基苯基)环氧乙烷-2-甲酸甲酯
CAS:42245-42-1 |
C11H12O4 |
|
Optimization of Serratia marcescens lipase production for en...
2004-12-01 [J. Ind. Microbiol. Biotechnol. 31(11) , 525-30, (2004)] |
|
A practical procedure for the large-scale preparation of met...
2002-06-28 [J. Org. Chem. 67(13) , 4599-601, (2002)] |
|
Occupational contact dermatitis from trans-methyl-3-(4-metho...
1991-10-01 [Contact Dermatitis 25(4) , 262-3, (1991)] |
|
Dermatitis from methyl 2,3 epoxy-3-(4-methoxyphenyl)propiona...
1990-11-01 [Contact Dermatitis 23(5) , 382, (1990)] |
|
Leukocytosis and low serum IgA in workers exposed to the epo...
1988-01-01 [Int. Arch. Occup. Environ. Health 60(1) , 7-14, (1988)] |