Short communication: A comparative analysis of recombinant chymosins.
J A Vallejo, J M Ageitos, M Poza, T G Villa
文献索引:J. Dairy Sci. 95(2) , 609-13, (2012)
全文:HTML全文
摘要
The first step in cheesemaking is the milk clotting process, in which κ-caseinolytic enzymes contribute to micelle precipitation. The best enzyme for this purpose is chymosin because of its high degree of specificity toward κ-casein. Although recombinant bovine chymosin is the most frequently used chymosin in the industry, new sources of recombinant chymosin, such as goat, camel, or buffalo, are now available. The present work represents a comparative study of 4 different recombinant chymosins (goat and buffalo chymosins expressed in Pichia pastoris, and bovine and camel chymosin expressed in Aspergillus niger). Recombinant goat chymosin exhibited the best catalytic efficiency compared with the buffalo, bovine, or camel recombinant enzymes. Moreover, recombinant goat chymosin exhibited the best specific proteolytic activity, a wider pH range of action, and a lower glycosylation degree than the other 3 enzymes. In conclusion, we propose that recombinant goat chymosin represents a serious alternative to recombinant bovine chymosin for use in the cheesemaking industry.Copyright © 2012 American Dairy Science Association. Published by Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
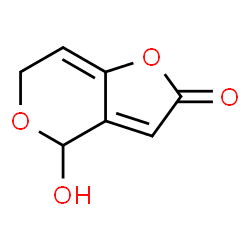 |
凝乳酶
CAS:9001-98-3 |
C7H6O4 |
|
Proteomic profiling of the coagulation of milk proteins indu...
2012-02-29 [J. Agric. Food Chem. 60(8) , 2039-45, (2012)] |
|
Starch addition in renneted milk gels: Partitioning between ...
2012-12-01 [J. Dairy Sci. 95(12) , 6871-81, (2012)] |
|
Constitutive expression, purification and characterization o...
2012-05-01 [World J. Microbiol. Biotechnol. 28(5) , 2087-93, (2012)] |
|
A regular curd consumption improves gastrointestinal status ...
2013-09-01 [Int. J. Food Sci. Nutr. 64(6) , 674-81, (2013)] |
|
A review on prorennin and rennin.
1966-01-01 [C. R. Trav. Lab. Carlsberg 35(8) , 143-231, (1966)] |