| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
二甲基亚砜
CAS:67-68-5 |
|
 |
水
CAS:7732-18-5 |
|
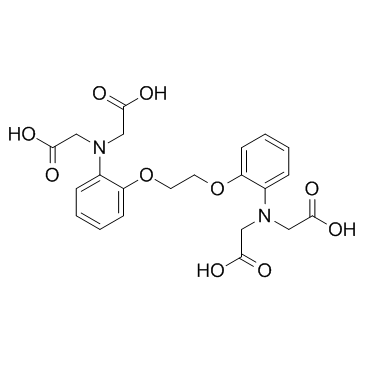 |
1,2-双(2-氨基苯氧基)乙烷-N,N,N',N'-四乙酸
CAS:85233-19-8 |
|
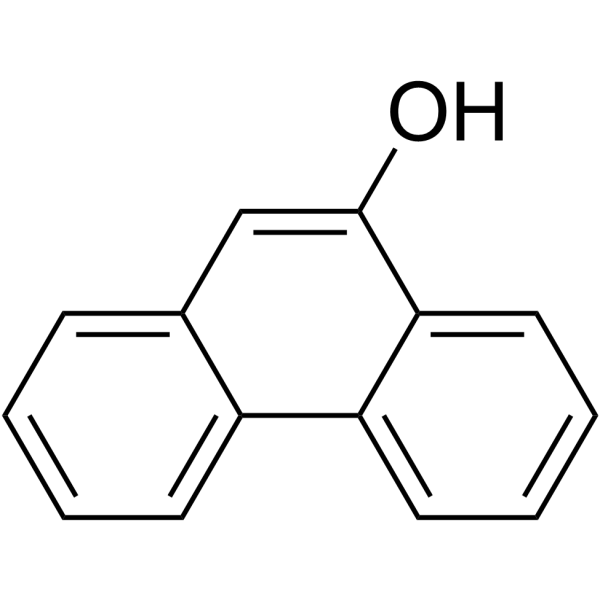 |
9-菲酚
CAS:484-17-3 |