| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
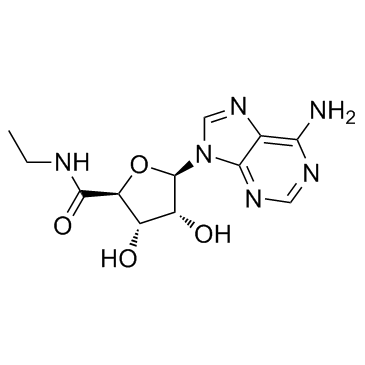 |
5'-N-乙基酰胺基腺苷
CAS:35920-39-9 |
|
 |
2-氯-N6-环戊基腺苷
CAS:37739-05-2 |