| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
L-赖氨酸盐酸盐
CAS:657-27-2 |
|
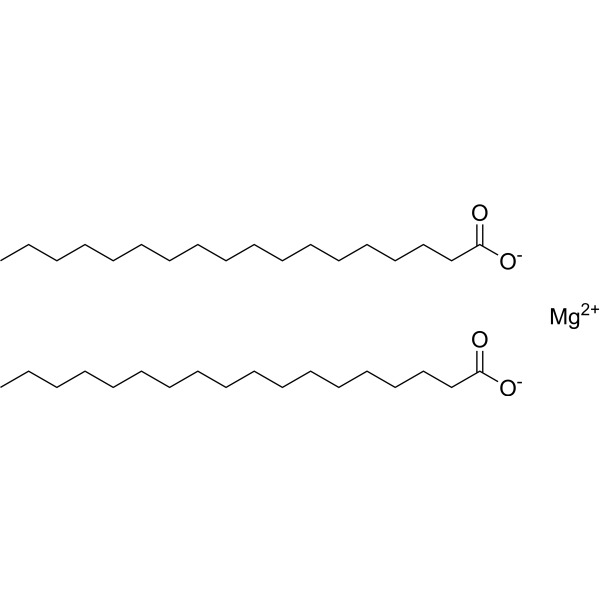 |
硬脂酸镁
CAS:557-04-0 |
|
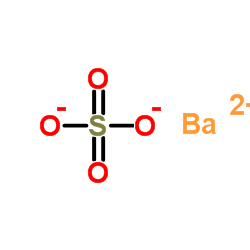 |
硫酸钡
CAS:7727-43-7 |
|
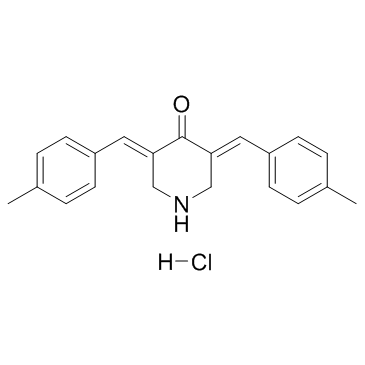 |
NSC 632839
CAS:157654-67-6 |
|
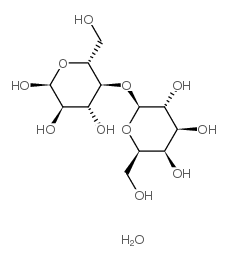 |
D-(+)-乳糖-水合物
CAS:64044-51-5 |