Origins of saccharide-dependent hydration at aluminate, silicate, and aluminosilicate surfaces.
Benjamin J Smith, Aditya Rawal, Gary P Funkhouser, Lawrence R Roberts, Vijay Gupta, Jacob N Israelachvili, Bradley F Chmelka
文献索引:Proc. Natl. Acad. Sci. U. S. A. 108(22) , 8949-54, (2011)
全文:HTML全文
摘要
Sugar molecules adsorbed at hydrated inorganic oxide surfaces occur ubiquitously in nature and in technologically important materials and processes, including marine biomineralization, cement hydration, corrosion inhibition, bioadhesion, and bone resorption. Among these examples, surprisingly diverse hydration behaviors are observed for oxides in the presence of saccharides with closely related compositions and structures. Glucose, sucrose, and maltodextrin, for example, exhibit significant differences in their adsorption selectivities and alkaline reaction properties on hydrating aluminate, silicate, and aluminosilicate surfaces that are shown to be due to the molecular architectures of the saccharides. Solid-state (1)H, (13)C, (29)Si, and (27)Al nuclear magnetic resonance (NMR) spectroscopy measurements, including at very high magnetic fields (19 T), distinguish and quantify the different molecular species, their chemical transformations, and their site-specific adsorption on different aluminate and silicate moieties. Two-dimensional NMR results establish nonselective adsorption of glucose degradation products containing carboxylic acids on both hydrated silicates and aluminates. In contrast, sucrose adsorbs intact at hydrated silicate sites and selectively at anhydrous, but not hydrated, aluminate moieties. Quantitative surface force measurements establish that sucrose adsorbs strongly as multilayers on hydrated aluminosilicate surfaces. The molecular structures and physicochemical properties of the saccharides and their degradation species correlate well with their adsorption behaviors. The results explain the dramatically different effects that small amounts of different types of sugars have on the rates at which aluminate, silicate, and aluminosilicate species hydrate, with important implications for diverse materials and applications.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
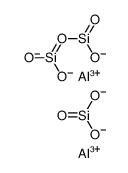 |
铝硅酸盐
CAS:1327-36-2 |
Al2H6O9Si3 |
|
Optical detection/collection of toxic Cd(II) ions using cubi...
2012-08-30 [Talanta 98 , 69-78, (2012)] |
|
Metal stabilization mechanism of incorporating lead-bearing ...
2012-02-01 [Chemosphere 86(8) , 817-21, (2012)] |
|
Ordered hexagonal mesoporous aluminosilicates with low Si/Al...
2014-06-01 [J. Nanosci. Nanotechnol. 14(6) , 4584-9, (2014)] |
|
The internal microstructure and fibrous mineralogy of fly as...
2011-12-01 [Environ. Pollut. 159(12) , 3324-33, (2011)] |
|
Inorganic/organic (SiO₂)/PEO hybrid electrospun nanofibers p...
2011-09-01 [ACS Appl. Mater. Interfaces 3(9) , 3673-81, (2011)] |