| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
鸟苷-5'-二磷酸葡萄糖酸钠盐
CAS:103301-72-0 |
|
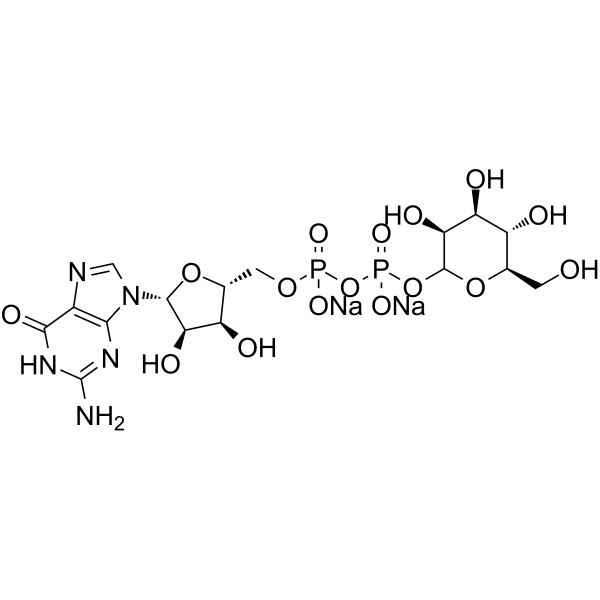 |
GDP-Mannose二钠盐
CAS:103301-73-1 |
|
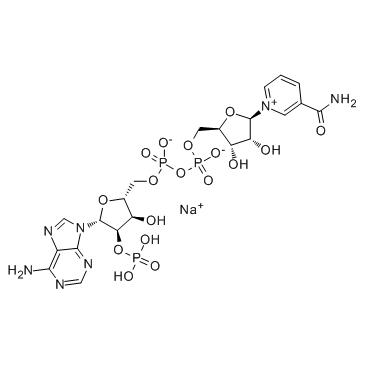 |
β-烟酰胺腺嘌呤二核苷酸磷酸钠盐
CAS:1184-16-3 |