Dual-function cinchona alkaloid catalysis: catalytic asymmetric tandem conjugate addition-protonation for the direct creation of nonadjacent stereocenters.
Yi Wang, Xiaofeng Liu, Li Deng
文献索引:J. Am. Chem. Soc. 128(12) , 3928-30, (2006)
全文:HTML全文
摘要
Catalytic tandem asymmetric reactions constitute a powerful strategy for the asymmetric construction of nonadjacent stereocenters in acyclic molecules directly from achiral precursors. In this Communication, we report a highly enantioselective and diastereoselective addition of trisubstituted carbon donors to 2-chloroacrylonitrile catalyzed by bifunctional cinchona alkaloid catalysts. This represents the first asymmetric tandem conjugate addition-protonation with efficient catalytic control of two nonadjacent stereocenters. As demonstrated in a concise and highly stereoselective formal total synthesis of (-)-manzacidin A, this asymmetric tandem reaction establishes a new and versatile catalytic approach for the enantioselective and diastereoselective creation of 1,3-tertiary-quaternary stereocenters.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
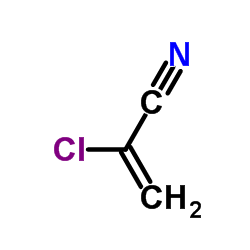 |
2-氯丙烯腈
CAS:920-37-6 |
C3H2ClN |
|
Structure-nephrotoxicity relationships of glutathione pathwa...
1989-05-31 [Toxicology 56(1) , 47-61, (1989)] |
|
[Information from the Soviet Toxicology Center].
1983-02-01 [Gig. Tr. Prof. Zabol. (2) , 52-3, (1983)] |
|
Asymmetric conjugate addition of oxindoles to 2-chloroacrylo...
2010-12-27 [Chemistry 16(48) , 14290-4, (2010)] |
|
Genotoxicity of 2-halosubstituted enals and 2-chloroacryloni...
1994-10-01 [Mutat. Res. 322(4) , 321-8, (1994)] |
|
Comparative developmental toxicities of aliphatic nitriles: ...
2000-03-01 [Toxicol. Appl. Pharmacol. 163(2) , 149-63, (2000)] |