Failure to demonstrate effectiveness of an anticholinergic drug in the symptomatic treatment of acute travelers' diarrhea.
R Reves, P Bass, H L DuPont, P Sullivan, J Mendiola
文献索引:J. Clin. Gastroenterol. 5(3) , 223-7, (1983)
全文:HTML全文
摘要
Seventy adults in the United States with acute diarrhea who were attending classes in Guadalajara, Mexico, enrolled in a double-blind placebo-controlled treatment study of an anticholinergic drug, mepenzolate bromide (MZB). Thirty-five patients received MZB (50 mg) and 35 received placebo each taken 4 times daily for 48 hours. No significant difference was detected between the MZB- and placebo-treated patients in symptoms or in the frequency or character of stools. Recovery rates of 24.1% and 31% for placebo- and MZB-treated patients were similar. Despite the occurrence of anticholinergic side effects in 51% of MZB- versus 14% of placebo-treated patients (P less than 0.001), therapeutic efficacy was not detected. We do not recommend anticholinergic drugs for therapy in acute infectious diarrhea.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
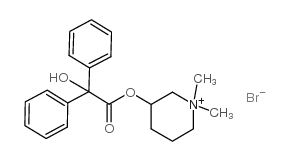 |
溴美喷酯
CAS:76-90-4 |
C21H26BrNO3 |
|
Ameliorative effect of mepenzolate bromide against pulmonary...
2014-07-01 [J. Pharmacol. Exp. Ther. 350(1) , 79-88, (2014)] |
|
Inflammation stimulates niacin receptor (GPR109A/HCA2) expre...
2014-12-01 [J. Lipid Res. 55(12) , 2501-8, (2014)] |
|
Muscarinic cholinergic receptors in human narcolepsy: a PET ...
1998-11-01 [Neurology 51(5) , 1297-302, (1998)] |
|
[Effect of tiemonium iodide on colonic motility in dogs].
1982-12-01 [Nippon. Yakurigaku Zasshi. 80(6) , 495-503, (1982)] |
|
Interaction of the antiarrhythmic drug amiodarone with the m...
1984-01-01 [J. Cardiovasc. Pharmacol. 6(6) , 1148-55, (1984)] |