| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
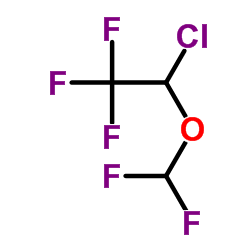 |
异氟醚
CAS:26675-46-7 |
|
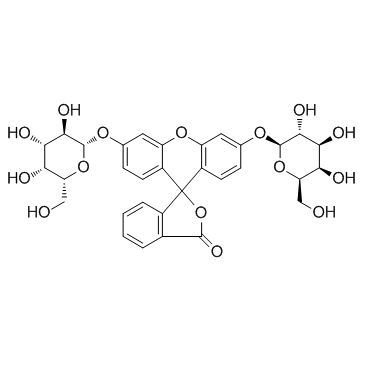 |
荧光素二(β-D-吡喃半乳糖苷)
CAS:17817-20-8 |
|
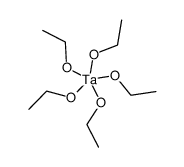 |
乙醇钽(V)
CAS:6074-84-6 |
|
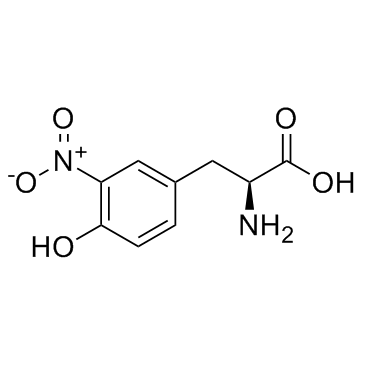 |
3-硝基-L-酪氨酸
CAS:621-44-3 |