Journal of the American Chemical Society
2008-08-06
Distinct reactivity of Pd(OTs)2: the intermolecular Pd(II)-catalyzed 1,2-carboamination of dienes.
Chris E Houlden, Chris D Bailey, J Gair Ford, Michel R Gagné, Guy C Lloyd-Jones, Kevin I Booker-Milburn
文献索引:J. Am. Chem. Soc. 130(31) , 10066-7, (2008)
全文:HTML全文
摘要
A Pd-catalyzed intermolecular 1,2-carboamination route to indolines from N-aryl ureas and 1,3-dienes that proceeds under mild conditions in relatively nonacidic media, is presented. The in situ generation, or preformation, of a palladium tosylate emerges as a key parameter in gaining the requisite reactivity for the C-H insertion/carbopalladation/nucleophilic displacement process.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
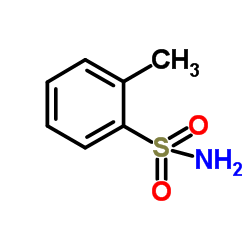 |
邻甲苯磺酰胺
CAS:88-19-7 |
C7H9NO2S |
相关文献:
更多...
|
Determination of o-toluenesulfonamide in artificial sweetene...
1978-11-01 [J. Assoc. Off. Anal. Chem. 61(6) , 1528-32, (1978)] |
|
The allergens of nail polish. (I). Allergenic constituents o...
1995-09-01 [Contact Dermatitis 33(3) , 157-64, (1995)] |
|
Mutagenicity study of Remsen-Fahlberg saccharin and contamin...
1980-11-01 [Toxicol. Lett. 7(1) , 51-60, (1980)] |
|
Toxicology of saccharin.
1984-10-01 [Fundam. Appl. Toxicol. 4(5) , 674-85, (1984)] |
|
Behaviour and biodegradation of sulfonamides (p-TSA, o-TSA, ...
2008-04-01 [Chemosphere 71(8) , 1574-81, (2008)] |