Lipase-catalyzed kinetic resolution on solid-phase via a "capture and release" strategy.
Cara E Humphrey, Nicholas J Turner, Morag A M Easson, Sabine L Flitsch, Rein V Ulijn
文献索引:J. Am. Chem. Soc. 125(46) , 13952-3, (2003)
全文:HTML全文
摘要
The lipase-catalyzed kinetic resolution of (R/S)-3-phenylbutyric acid 2 using solid-supported cyclohexane-1,3-dione (CHD) 6 is described. In each case the predominant enantiomer observed, after cleavage from the resin, was (R)-(-)-3-phenylbutyric acid (R)-2 (ee > 99%) rather than the expected (S)-enantiomer of 2. This observation is in contrast to the fact that Chromobacterium viscosum lipase shows high enantiospecificity for the (S)-enantiomer in the corresponding solution-phase hydrolysis reactions. The (R)-acyl group was subsequently released from the resin by NaOH hydrolysis, and the yield of the reaction could be improved by triple acylation of the resin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
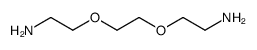 |
聚氧乙烯二胺
CAS:24991-53-5 |
(C2H4O)nC4H12N2O |
|
Synthesis of semitelechelic maleimide poly(PEGA) for protein...
2009-07-13 [Biomacromolecules 10(7) , 1777-81, (2009)] |
|
Construction of highly glycosylated mucin-type glycopeptides...
2006-04-14 [J. Org. Chem. 71(8) , 3051-63, (2006)] |
|
Solid-phase library synthesis, screening, and selection of t...
2002-05-09 [J. Med. Chem. 45(10) , 1971-82, (2002)] |
|
Improved biotransformations on charged PEGA supports.
2003-06-07 [Chem. Commun. (Camb.) (11) , 1296-7, (2003)] |
|
Characterization of modified alginate-poly-L-lysine microcap...
1997-09-01 [Artif. Organs 21(9) , 1002-6, (1997)] |