New compounds obtained by evolution and oxidation of malvidin 3-O-glucoside in ethanolic medium.
Nour-Eddine Es-Safi, Emmanuelle Meudec, Claire Bouchut, Helene Fulcrand, Paul-Henri Ducrot, Gaëtan Herbette, Veronique Cheynier
文献索引:J. Agric. Food Chem. 56(12) , 4584-91, (2008)
全文:HTML全文
摘要
Two new colorless phenolic compounds were formed from malvidin 3- O-glucoside incubated in an ethanolic solution. Their structures were characterized by means of one- and two-dimensional NMR analysis and through electrospray ionization-mass spectrometry. As compared to the structure of the initial anthocyanin skeleton, the first new compound showed the presence of two fused five-membered rings replacing the pyran ring and of a carbonyl function on the 2-position. The first five-membered ring was shown to result from the formation of a new linkage between the B ring 6'-position and the C ring 4-position, while the second was a dihydro furan ring with an oxygenated ether linkage between the 8a-position and the 3-position. The second isolated compound was shown to have similar structure with an ethyl ether moiety in the 3-position instead of the glucose moiety. A mechanism explaining the formation of the isolated compounds involving the passage through the chalcone form of the anthocyanin and an oxidation process is proposed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
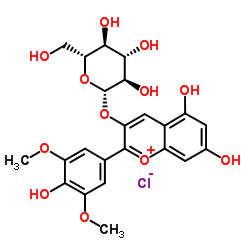 |
氯化锦葵色素-3-O-葡萄糖苷
CAS:7228-78-6 |
C23H25ClO12 |
|
The identification of degradation products and degradation p...
2013-12-01 [Food Chem. 141(3) , 3260-7, (2013)] |
|
Malvidin-3-glucoside protects endothelial cells up-regulatin...
2012-09-30 [Chem. Biol. Interact. 199(3) , 192-200, (2012)] |
|
Comprehensive colorimetric study of anthocyanic copigmentati...
2012-03-21 [J. Agric. Food Chem. 60(11) , 2896-905, (2012)] |
|
Induction of apoptosis and inhibition of invasion in human h...
2009-08-01 [Ann. N. Y. Acad. Sci. 1171 , 137-48, (2009)] |
|
Principal components of phenolics to characterize redVinho V...
2008-01-01 [Talanta 75(5) , 1190-202, (2008)] |