| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
酪胺盐酸盐
CAS:59880-97-6 |
|
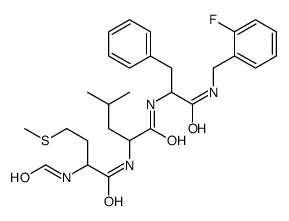 |
N-FORMYL-MET-LEU-PHE-O-FLUOROBENZYLAMIDE
CAS:112898-02-9 |