Synthetic approaches to indolo[6,7-a]pyrrolo[3,4-c]carbazoles: potent cyclin D1/CDK4 inhibitors.
Margaret M Faul, Thomas A Engler, Kevin A Sullivan, John L Grutsch, Marcella T Clayton, Michael J Martinelli, Joseph M Pawlak, Michael LeTourneau, D Scott Coffey, Steven W Pedersen, Stanley P Kolis, Kelly Furness, Sushant Malhotra, Rima S Al-awar, James E Ray
文献索引:J. Org. Chem. 69 , 2967-2975, (2004)
全文:HTML全文
摘要
Synthesis of indolo[6,7-a]pyrrolo[3,4-c]carbazoles 1, a new class of cyclin D1/CDK4 inhibitors, by oxidation of the corresponding aryl indolylmaleimides 2, will be described. Two approaches to the synthesis of 2 were identified that required new methods for the synthesis of 7-substituted indole acetamides 3 and N-methyl (indol-7-yl)oxoacetates 6. The chemistry developed enabled introduction of functionality (-OR, NR(2)) at C(12) and N(13) facilitating structure-activity relationship (SAR) evaluation of this indolocarbazole platform.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
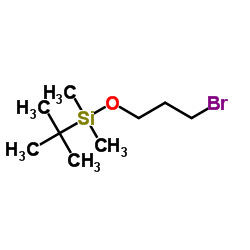 |
3-溴丙基叔丁基二甲基硅醚
CAS:89031-84-5 |
C9H21BrOSi |
|
Synthesis and selective cyclooxygenase-2 inhibitory activity...
2004-04-22 [J. Med. Chem. 47 , 2180-2193, (2004)] |
|
A concise total synthesis of (+/-)-vigulariol.
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46 , 437, (2007)] |
|
3-(7-Azaindolyl)-4-arylmaleimides as potent, selective inhib...
2004-06-21 [Bioorg. Med. Chem. Lett. 14 , 3245-3250, (2004)] |
|
Synthesis, radioiodination and in vivo screening of n...
[European J. Org. Chem. 63 , 840-853, (2013)] |
|
Synthesis of O-(3-[18F] Fluoropropyl)-L-ty...
[Bull. Korean Chem. Soc. 26(1) , 91-96, (2005)] |