| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
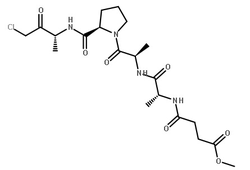 |
弹性蛋白酶,来源猪胰腺
CAS:39445-21-1 |
|
 |
α-糜蛋白酶
CAS:9004-07-3 |
|
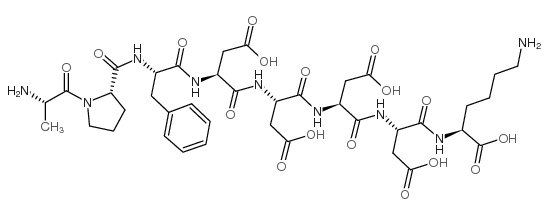 |
胰蛋白酶原
CAS:9002-08-8 |