Pharmacokinetics and metabolism of allopurinol riboside.
T A Shapiro, J B Were, K Danso, D J Nelson, R E Desjardins, C L Pamplin
文献索引:Clin. Pharmacol. Ther. 49(5) , 506-14, (1991)
全文:HTML全文
摘要
There are no safe and effective oral drugs to treat leishmaniasis and Chagas' disease. The safety, pharmacokinetics, and metabolism of single and multiple oral doses of allopurinol riboside, an investigational antiparasitic agent, were evaluated in a randomized, double-blinded, placebo-controlled study in 32 healthy male volunteers, at levels up to 25 mg/kg q.i.d. for 13 doses. No significant toxicity was detected. Allopurinol riboside peaks in plasma 1.6 hours after administration, has an elimination half-life of 3 hours, and steady-state concentrations in the therapeutic range. However, in contrast to preclinical studies in dogs (plasma levels proportional to oral doses up to 200 mg/kg), we found that plasma levels were unexpectedly low and did not rise with increasing dose. Furthermore, allopurinol and oxypurinol (unanticipated metabolites) were detected at levels proportional to the dose of allopurinol riboside. We present a model that includes incomplete absorption, metabolism of residual drug by enteric flora, and absorption of bacterial metabolites to explain these findings in humans.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
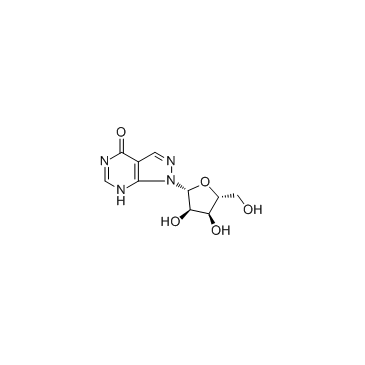 |
别嘌呤醇核糖苷
CAS:16220-07-8 |
C10H12N4O5 |
|
Activity of inosine analogs against Pneumocystis carinii in ...
1986-07-01 [Antimicrob. Agents Chemother. 30(1) , 181-3, (1986)] |
|
A tissue culture system for the growth of several species of...
1988-03-01 [Am. J. Trop. Med. Hyg. 38(2) , 304-7, (1988)] |
|
On the metabolism of allopurinol. Formation of allopurinol-1...
1983-07-15 [Biochem. Pharmacol. 32(14) , 2167-74, (1983)] |
|
Efficacy of pyrazolopyrimidine ribonucleosides against Trypa...
1984-10-01 [J. Infect. Dis. 150(4) , 602-8, (1984)] |
|
In vitro antileishmanial activity of inhibitors of steroid b...
1987-06-01 [J. Parasitol. 73(3) , 671-3, (1987)] |