| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
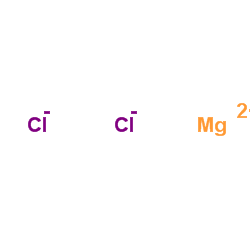 |
氯化镁
CAS:7786-30-3 |
|
 |
荧光素酶 来源于萤火虫
CAS:61970-00-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
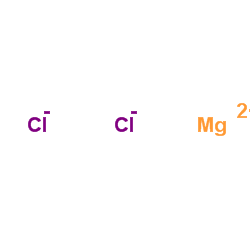 |
氯化镁
CAS:7786-30-3 |
|
 |
荧光素酶 来源于萤火虫
CAS:61970-00-1 |