| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
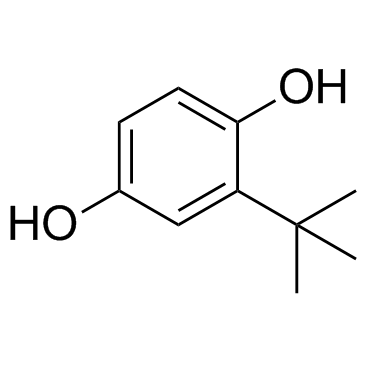 |
叔丁基对苯二酚(TBHQ)
CAS:1948-33-0 |
|
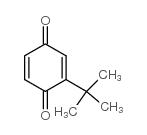 |
叔丁基对苯醌
CAS:3602-55-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
叔丁基对苯二酚(TBHQ)
CAS:1948-33-0 |
|
 |
叔丁基对苯醌
CAS:3602-55-9 |