Aztreonam lysine: a novel inhalational antibiotic for cystic fibrosis.
Michael D Parkins, J Stuart Elborn
文献索引:Expert Rev. Respir. Med. 4(4) , 435-44, (2010)
全文:HTML全文
摘要
Acquisition of Pseudomonas aeruginosa, the most prevalent organism isolated from cystic fibrosis (CF) airways, is associated with an accelerated clinical deterioration and reduced survival. Strategies to chronically suppress P. aeruginosa infections in individuals with CF have evolved over the last four decades and now largely focus on regular administration of aerosolized antibiotics. Aztreonam lysine (AZLI; Cayston, Gilead Pharmaceuticals [Foster City, CA, USA]), a novel formulation of the monobactam aztreonam suitable for aerosol delivery has recently been developed. AZLI is administered as 75 mg three-times daily for 28 days in 'on/off' cycles using the Altera/eFlow electronic nebulizer (PARI Innovative Manufacturers [Midlothian, VA, USA]). In individuals with CF chronically infected with P. aeruginosa, AZLI improved healthcare-associated quality-of-life scores, pulmonary function and weight, prolonged time to requirement of antibacterial therapy for symptoms of pulmonary exacerbation and reduced P. aeruginosa sputum burdens. These outcomes were durable over 18 months of cycled use. AZLI has been demonstrated to be safe and effective, and expands available chronic maintenance therapies in CF.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
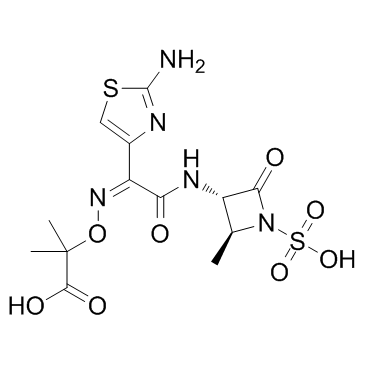 |
氨曲南
CAS:78110-38-0 |
C13H17N5O8S2 |
|
Tolerability of aztreonam and carbapenems in patients with I...
2015-04-01 [J. Allergy Clin. Immunol. 135(4) , 972-6, (2015)] |
|
In vitro antibacterial activity of AZD0914, a new spiropyrim...
2015-01-01 [Antimicrob. Agents Chemother. 59(1) , 467-74, (2014)] |
|
An in vitro deletion in ribE encoding lumazine synthase cont...
2014-12-01 [Antimicrob. Agents Chemother. 58(12) , 7225-33, (2014)] |
|
LTX-109 is a novel agent for nasal decolonization of methici...
2015-01-01 [Antimicrob. Agents Chemother. 59(1) , 145-51, (2014)] |
|
An outbreak of blaOXA-51-like- and blaOXA-66-positive Acinet...
2014-11-01 [J. Med. Microbiol. 63(Pt 11) , 1517-23, (2014)] |