| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
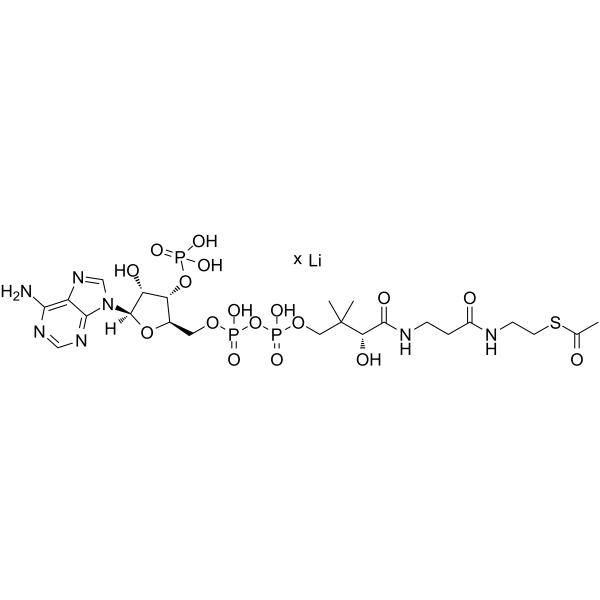 |
乙酰辅酶A三锂盐
CAS:32140-51-5 |
|
 |
乙酰辅酶 A 钠盐
CAS:102029-73-2 |
|
 |
乙酰辅酶A合成酶 来源于面包酵母(酿酒酵母)
CAS:9012-31-1 |