Influence of a 4-aminomethylbenzoic acid residue on competitive fragmentation pathways during collision-induced dissociation of metal-cationized peptides.
Sandra Osburn, Sila Ochola, Erach Talaty, Michael Van Stipdonk
文献索引:Rapid Commun. Mass Spectrom. 21(21) , 3409-19, (2007)
全文:HTML全文
摘要
Formation of [bn+17+cat]+ is a prominent collision-induced dissociation (CID) pathway for Li+- and Na+-cationized peptides. Dissociation of protonated and Ag+-cationized peptides instead favors formation of the rival bn+/[bn-1+cat]+ species. In this study the influence of a 4-aminomethylbenzoic acid (4AMBz) residue on the relative intensities of [b(3)-1+cat]+ and [b(3)+17+cat]+ fragment ions was investigated using several model tetrapeptides including those with the general formula A(4AMBz)AX and A(4AMBz)GX (where X=G, A, V). For Li+- and Na+-cationized versions of the peptides there was a significant increase in the intensity of [b(3)-1+cat]+ for the peptides that contain the 4AMBz residue, and in some cases the complete elimination of the [b(3)+17+cat]+ pathway. The influence of the 4AMBz residue may be attributed to the fact that [b(3)-1+cat]+ would be a highly conjugated species containing an aromatic ring substituent. Comparison of CID profiles generated from Na+-cationized AAGV and A(4AMBz)GV suggests an apparent decrease in the critical energy for generation of [b(3)-1+Na]+ relative to that of [b(3)+17+Na]+ when the aromatic amino acid occupies a position such that it leads to the formation of the highly conjugated oxazolinone, thus leading to an increase in formation rate for the former compared to the latter.Copyright (c) 2007 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
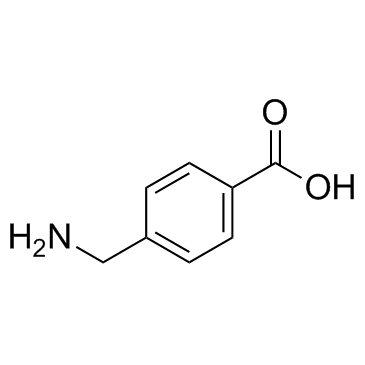 |
氨甲苯酸
CAS:56-91-7 |
C8H9NO2 |
|
Substituent effects on the energetics and aromaticity of ami...
2007-10-25 [J. Phys. Chem. A 111(42) , 10598-603, (2007)] |
|
Ligand specificity of human plasminogen kringle 4.
1991-11-19 [Biochemistry 30(46) , 11081-92, (1991)] |
|
Spectrophotometric determination of aminomethylbenzoic acid ...
2007-03-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(3) , 656-61, (2007)] |
|
The effect of intravesical instillation of antifibrinolytic ...
2008-04-01 [J. Urol. 179(4) , 1307-11; discussion 1311-2, (2008)] |
|
Study on enhancement of fibronectin-mediated bacillus Calmet...
2007-10-01 [World J. Urol. 25(5) , 525-9, (2007)] |