Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1Aactivity, Part 1
Franciszek Herold, Andrzej Chodkowski, Łukasz Izbicki, Marek Król, Jerzy Kleps, Jadwiga Turło, Gabriel Nowak, Katarzyna Stachowicz, Małgorzata Dybała, Agata Siwek, Franciszek Herold, Andrzej Chodkowski, Łukasz Izbicki, Marek Król, Jerzy Kleps, Jadwiga Turło, Gabriel Nowak, Katarzyna Stachowicz, Małgorzata Dybała, Agata Siwek
文献索引:Eur. J. Med. Chem. 44 , 1710-7, (2009)
全文:HTML全文
摘要
A series of new derivatives of 4-aryl-pyrido[1,2- c]pyrimidine containing the 3-(4-piperidyl)-1 H-indole residue or its 5-methoxy derivative were synthesized. They were characterized ( i) in vitro by binding to 5-HT 1A receptors and 5-HT transporter proteins in rat brain cortex membranes and ( ii) in vivo in the mouse by induced hypothermia and forced swimming models for antagonist/agonist activity against the 5-HT 1A autoreceptors and postsynaptic 5-HT 1A receptors, respectively. Structure activity relationship evaluation indicated that the presence of the 3-(4-piperidyl)-1 H-indole residue and ortho- or para-substituents with –F or –CH 3 groups in the aryl ring as well as an unsubstituted aryl in the 4-aryl-pyrido[1,2- c]pyrimidine moiety promoted low K i values for both receptors. In contrast, the presence of a 5-methoxy-3-(4-piperidyl)-1 H-indole residue as well as –Cl or –OCH 3 substituents at the para position markedly reduced the receptor affinity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
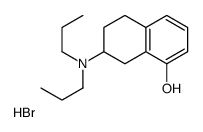 |
(±)-8-羟基-2-(二丙基氨基)四氢萘 氢溴酸盐
CAS:87394-87-4 |
C16H26BrNO |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
5-HT1D receptor inhibits renal sympathetic neurotransmission...
2015-09-01 [Vascul. Pharmacol. 72 , 172-80, (2015)] |
|
Pharmacological evidence that 5-HT1D activation induces rena...
2015-06-01 [Clin. Exp. Pharmacol. Physiol. 42 , 640-7, (2015)] |
|
Serotonin 5-HT1A receptor in infancy-onset aggression: compa...
2013-04-15 [Behav. Brain Res. 243 , 97-101, (2013)] |
|
Synthesis and biological investigation of potential atypical...
2011-09-01 [Eur. J. Med. Chem. 46 , 4474-88, (2011)] |