| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
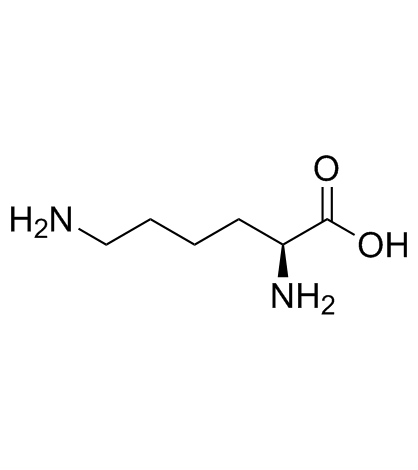 |
L-赖氨酸
CAS:56-87-1 |
|
 |
L-赖氨酸盐酸盐
CAS:657-27-2 |
|
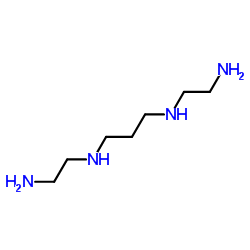 |
N,N'-二(2-氨乙基)-1,3-丙二胺
CAS:4741-99-5 |
|
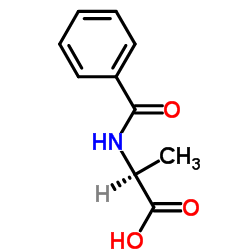 |
N-苯甲酰-L-丙氨酸
CAS:2198-64-3 |
|
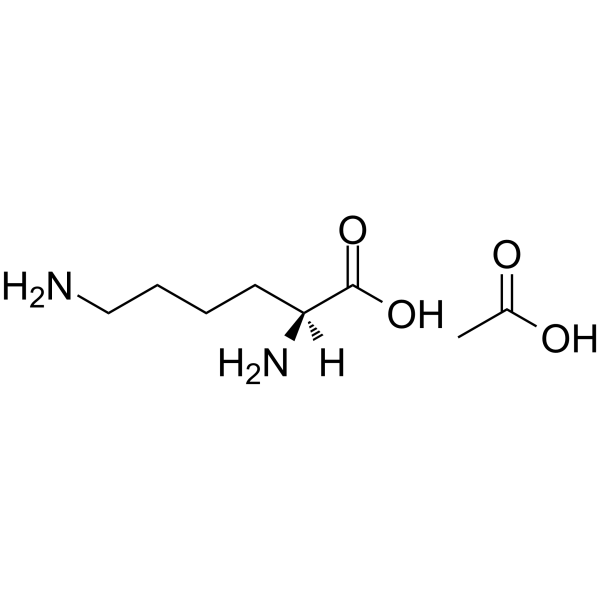 |
醋酸赖氨酸
CAS:57282-49-2 |