| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
1,2-二氯乙烷
CAS:107-06-2 |
|
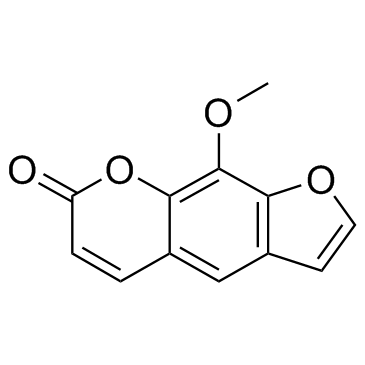 |
花椒毒素; 8-甲氧基补骨脂素
CAS:298-81-7 |
|
 |
甲磺司特
CAS:94055-76-2 |
|
 |
3,4-二氢-2H-吡喃
CAS:110-87-2 |
|
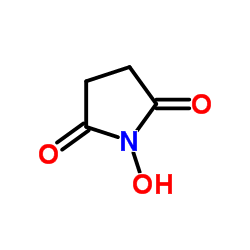 |
N-羟基琥珀酰亚胺
CAS:6066-82-6 |