| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
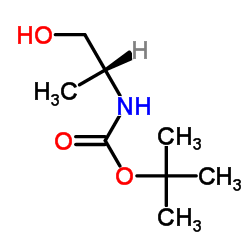 |
BOC-D-丙氨醇
CAS:106391-86-0 |
|
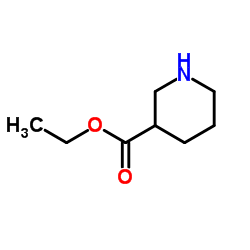 |
3-哌啶甲酸乙酯
CAS:5006-62-2 |
|
 |
α,α'-二溴对二甲苯
CAS:623-24-5 |