| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
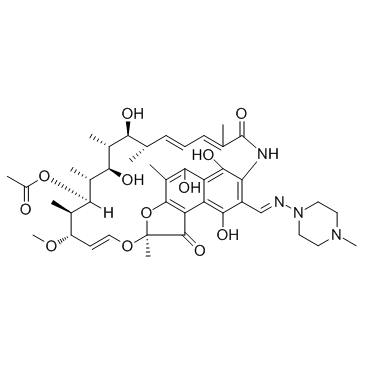 |
利福平
CAS:13292-46-1 |
|
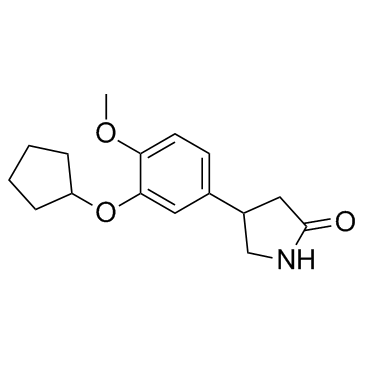 |
咯利普兰
CAS:61413-54-5 |
|
 |
西地那非
CAS:139755-83-2 |