| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
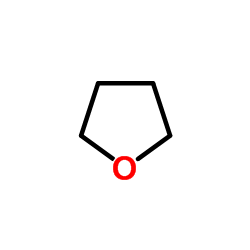 |
四氢呋喃
CAS:109-99-9 |
|
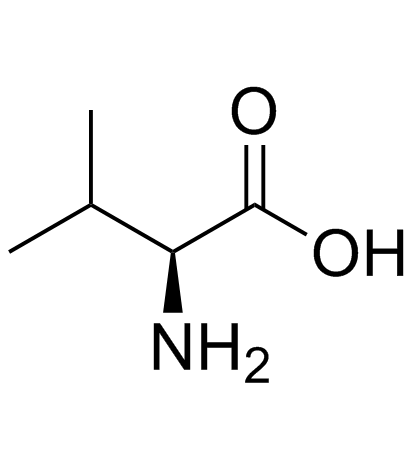 |
l-缬氨酸
CAS:72-18-4 |
|
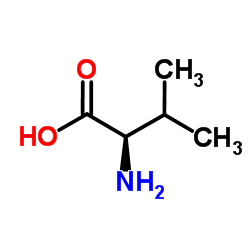 |
D-缬氨酸
CAS:640-68-6 |
|
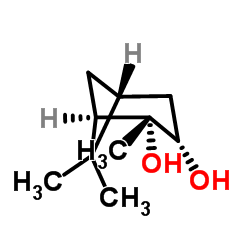 |
(1R,2R,3S,5R)-(-)-2,3-蒎烷二醇
CAS:22422-34-0 |