| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
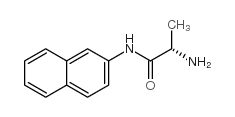 |
L-丙氨酸β-萘酰胺
CAS:720-82-1 |
|
 |
DL-氨基丙酸 β-氢氯化萘基酰胺
CAS:74144-49-3 |
|
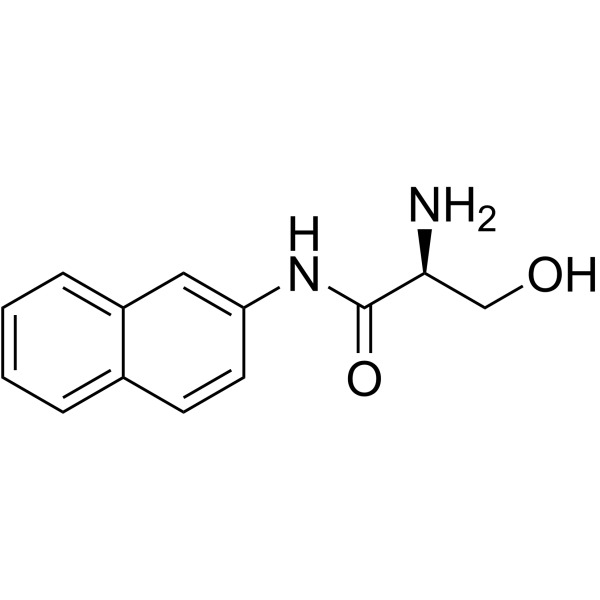 |
L-丝氨酸-beta-萘酰胺
CAS:888-74-4 |