Bioorthogonal proteomics of 15-hexadecynyloxyacetic acid chemical reporter reveals preferential targeting of fatty acid modified proteins and biosynthetic enzymes.
Jacob S Yount, Guillaume Charron, Howard C Hang
文献索引:Bioorg. Med. Chem. 20(2) , 650-4, (2012)
全文:HTML全文
摘要
Chemical reporters are powerful tools for the detection and discovery of protein modifications following cellular labeling. The metabolism of alkyne- or azide-functionalized chemical reporters in cells can influence the efficiency and specificity of protein targeting. To evaluate the effect of degradation of chemical reporters of protein fatty acylation, we synthesized 15-hexadecynyloxyacetic acid (HDYOA), a reporter that was designed to be resistant to β-oxidation, and compared its ability to label palmitoylated proteins with an established reporter, 17-octadecynoic acid (ODYA). HDYOA was able to label known candidate S-palmitoylated proteins similarly to ODYA. Accordingly, bioorthogonal proteomic analysis demonstrated that 70% of proteins labeled with ODYA were also labeled with HDYOA. However, the proteins observed differentially in our proteomic studies suggested that a portion of ODYA protein labeling is a result of β-oxidation. In contrast, downstream enzymes involved in β-oxidation of fatty acids were not targeted by HDYOA. Since HDYOA can label S-palmitoylated proteins and is not utilized by downstream β-oxidation pathways, this fatty acid chemical reporter may be particularly useful for bioorthogonal proteomic studies in cell types metabolically skewed toward fatty acid breakdown.Copyright © 2011 Elsevier Ltd. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
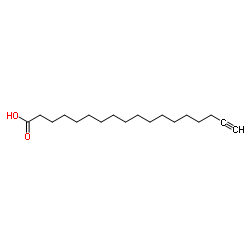 |
17-十八炔酸
CAS:34450-18-5 |
C18H32O2 |
|
Bioorthogonal mimetics of palmitoyl-CoA and myristoyl-CoA an...
2015-06-01 [Proteomics 15 , 2066-77, (2015)] |
|
Interaction of epoxyeicosatrienoic acids and adipocyte fatty...
2015-05-01 [Int. J. Obes. 39 , 755-61, (2015)] |
|
Species differences in intestinal metabolic activities of cy...
2011-06-01 [Drug Metab. Pharmacokinet. 26(3) , 300-6, (2011)] |
|
Arachidonic acid-dependent gene regulation during preadipocy...
2014-12-01 [J. Lipid Res. 55(12) , 2479-90, (2014)] |
|
Changes in renal haemodynamics induced by indomethacin in th...
2004-10-01 [Clin. Exp. Pharmacol. Physiol. 31(10) , 683-90, (2004)] |