| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
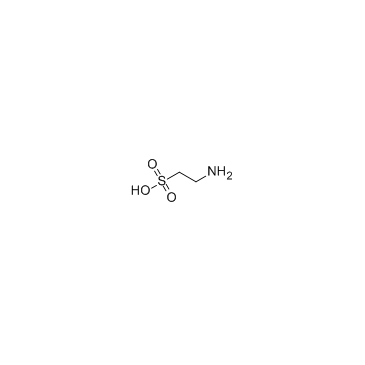 |
牛磺酸
CAS:107-35-7 |
|
 |
四氢嘧啶(依克多因)
CAS:96702-03-3 |
|
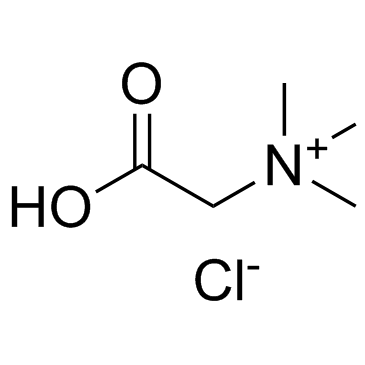 |
甜菜碱盐酸盐
CAS:590-46-5 |
|
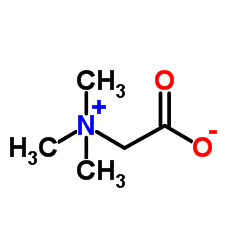 |
甜菜碱
CAS:107-43-7 |