[Study on the interaction between alginic sodium diester and azur A and the application].
Yong-ming Liu, Gui-zhi Li, Fei Pan
文献索引:Guang Pu Xue Yu Guang Pu Fen Xi 27(2) , 317-20, (2007)
全文:HTML全文
摘要
The interaction of alginic sodium diester (ASD) and azur A (AA) was studied by absorption spectra. The influence of pH and the molar ratio of AA/ASD on the spectra was investigated. The maximum binding number (N = 168) and the binding equilibrium constant (K = 3.25 x 10(6)) of the alginic sodium diester and azur A were obtained by the theoretical model. The new method for the determination of ASD was developed by the formation of ASD-AA ion-association complex that caused the fading of the AA. The detection limit is 0.009 microg x mL(-1). The method has the advantages of simplicity, stable system, good accuracy and selectivity, and was applied to the determination of alginic sodium diester in tablets with satisfactory results.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
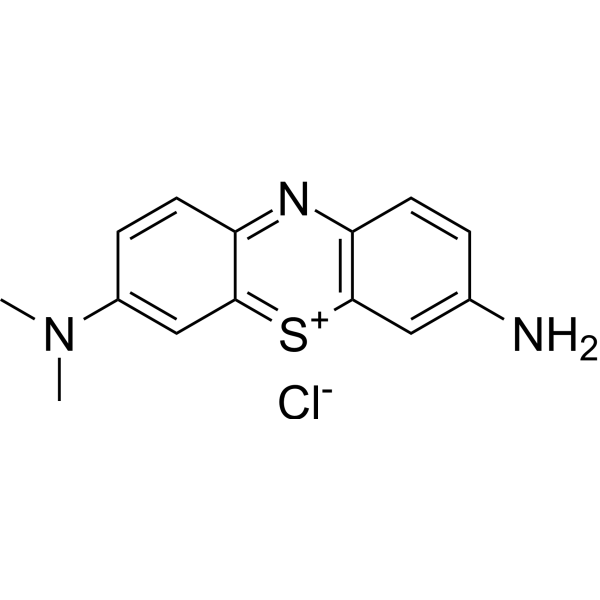 |
天青 A
CAS:531-53-3 |
C14H14ClN3S |
|
A chemical screening approach reveals that indole fluorescen...
2009-09-01 [Bioorg. Med. Chem. Lett. 19 , 4952-7, (2009)] |
|
Filamin-interacting proteins, Cfm1 and Cfm2, are essential f...
2014-06-01 [Hum. Mol. Genet. 23(11) , 2953-67, (2014)] |
|
Ligand polarizability contributes to tau fibril binding affi...
2011-09-01 [Bioorg. Med. Chem. 19 , 5147-54, (2011)] |
|
Effect of Excessive Potassium Iodide on Rat Aorta Endothelia...
2015-08-01 [Biol. Trace Elem. Res. 166 , 201-9, (2015)] |
|
Grafted Azure A modified electrodes as disposable β-nicotina...
2012-10-17 [Anal. Chim. Acta 747 , 84-91, (2012)] |