Phase II study of rubitecan in recurrent or metastatic head and neck cancer.
Francesco Caponigro, Giacomo Cartenì, Jean Pierre Droz, Amalia Milano, Wayne B Davis, Patricia Pollard
文献索引:Cancer Chemother. Pharmacol. 62(2) , 209-14, (2008)
全文:HTML全文
摘要
Rubitecan is an oral camptothecin analogue that has shown activity against a broad spectrum of human tumor xenografts and has been tested in several diseases.In the present study, 19 patients with incurable, recurrent or metastatic head and neck cancer were treated with rubitecan at the initial dose of 1.5 mg/m(2) x 5 days per week. An appropriate dose modification program was set up according to the observed toxicities.Thirteen out of the 19 treated patients were formally evaluable for tumor response. Ten patients had a disease progression and three patients had a stabilization of disease as their best response. The mean duration of stable disease was 141 days. Median survival was 16 weeks (range 2-22 weeks). Three patients died during the study or less than a month after their last dose of study medication. Hematologic toxicity was serious in this study since four patients discontinued their participation because of severe anemia. The drug was also associated with grade 1-4 neutropenia, and with 1-3 thrombocytopenia.We conclude that rubitecan is not effective as a single-agent in recurrent or metastatic head and neck cancer with the doses and schedule used in this study.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
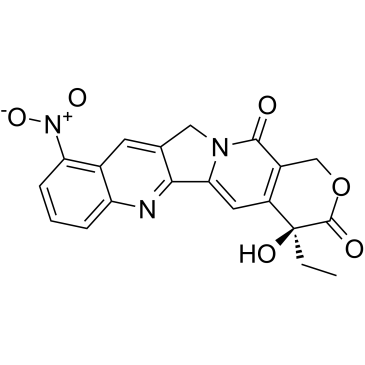 |
9-硝基喜树碱
CAS:91421-42-0 |
C20H15N3O6 |
|
Effect of injection routes on pharmacokinetics and lactone/c...
2007-08-01 [Int. J. Pharm. 340(1-2) , 29-33, (2007)] |
|
Effect of phospholipid composition on characterization of li...
2006-07-01 [Drug Dev. Ind. Pharm. 32(6) , 719-26, (2006)] |
|
Sequential oral 9-nitrocamptothecin and etoposide: a pharmac...
2006-08-01 [Mol. Cancer Ther. 5(8) , 2130-7, (2006)] |
|
9-nitrocamptothecin polymeric nanoparticles: cytotoxicity an...
2008-09-01 [Anticancer Drugs 19(8) , 805-11, (2008)] |
|
Anticancer activity of new haloalkyl camptothecin esters aga...
2012-09-01 [Anticancer Agents Med. Chem. 12(7) , 818-28, (2012)] |