Fluorescence spectroscopic investigation of the interaction between triphenyltin and humic acids.
Hong-Mei Zhang, Qiu-Hua Zhou, Mei-Gui Xue, Yan-Qing Wang
文献索引:Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 78(3) , 1018-22, (2011)
全文:HTML全文
摘要
The interaction between triphenyltin (TPT) and humic acid (HA) was investigated using UV-vis and fluorescence spectra techniques. The experimental results showed that the fluorescence quenching of HA by TPT was a result of the interaction of TPT with HA. The binding constant K(b) and corresponding thermodynamic parameters were measured at different temperatures. The binding of TPT molecule to HA is a spontaneous molecular interaction procedure in which entropy increased and Gibbs free energy decreased. Hydrophobic interaction force plays a major role in stabilizing the TPT-HA complex. The three-dimensional fluorescence contour spectra revealed that TPT could enter into the hydrophobic cavities in some domain of HA.Copyright © 2010 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
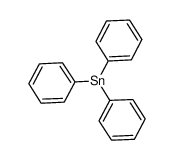 |
三苯基锡化氢
CAS:892-20-6 |
C18H15Sn |
|
Comparative toxicity of antifouling compounds on the develop...
2011-11-01 [Ecotoxicology 20(8) , 1870-80, (2011)] |
|
Effects of organotin alternative antifoulants on oyster embr...
2011-07-01 [Arch. Environ. Contam. Toxicol. 61(1) , 128-34, (2011)] |
|
Triphenyltin induced growth inhibition and antioxidative res...
2011-01-01 [Ecotoxicology 20(1) , 73-80, (2011)] |
|
Influence of triphenyltin exposure on the hypothalamus–pitui...
2011-01-01 [Aquat. Toxicol. 104(3-4) , 263-9, (2011)] |
|
Sex-dependent aromatase activity in rat offspring after pre-...
2010-10-29 [Toxicology 276(3) , 198-205, (2010)] |