| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氨基甲烷
CAS:74-89-5 |
|
 |
冰醋酸
CAS:64-19-7 |
|
 |
镍
CAS:7440-02-0 |
|
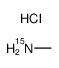 |
甲基胺-15N盐酸盐
CAS:3852-22-0 |
|
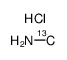 |
甲胺-13C 盐酸盐
CAS:60656-93-1 |
|
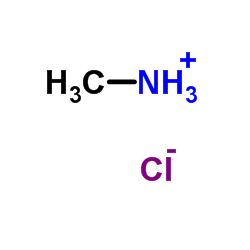 |
一甲胺盐酸盐
CAS:593-51-1 |