Purification and characterization of chymodenin. A hormone-like peptide from porcine duodenum.
J W Adelson, L Nelbach, G B Yates, A Ehrlich, C B Glaser, R Chang
文献索引:J. Biol. Chem. 261(23) , 10569-10575, (1986)
全文:HTML全文
摘要
Chymodenin, a hormone-like duodenal peptide which rapidly alters the proportions of secreted pancreatic digestive enzymes to a mixture relatively richer in chymotrypsinogen than that found in basal secretion, has been purified to homogeneity. The starting material was an acidic methanol-soluble, neutral pH-insoluble fraction of an acetic acid extract of porcine duodenum; the purification consisted of cation-exchange chromatography on SP-Sephadex and CM-Sephadex in ammonium bicarbonate gradients, and gel filtration on Sephadex G-75 in dilute acetic acid. The yield of material was followed by radioimmunoassay. Homogeneity was determined from chymodenin's behavior in disc gel electrophoresis in an acidic counter-migration-of-dye system, sodium dodecyl sulfate-urea gels, gel filtration, dansyl-Edman reaction, reversed-phase high pressure liquid chromatography, isotachophoresis, and sedimentation equilibrium ultracentrifugation. The electrophoretic mobility, the molecular weight of 9,000-10,000, and the biological activity differed from those of other gastrointestinal peptide hormones. The amino acid composition was unique. Chymodenin is the first purified hormone-like substance reported capable of altering the composition of the mixture of secreted digestive enzymes, independent of the stimulation of massive pancreatic protein output.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
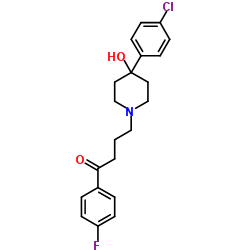 |
羧甲基葡聚糖凝胶C-25
CAS:62886-59-3 |
C21H23ClFNO2 |
|
Multiple modes of interaction between the methylated DNA bin...
2007-02-01 [Mol. Cell. Biol. 27(3) , 864-877, (2007)] |
|
A novel serine protease inhibitor from Bungarus fasciatus ve...
2008-03-01 [Peptides 29 , 369-374, (2008)] |
|
An anticoagulant serine protease from the wasp venom of Vesp...
2008-04-01 [Toxicon 51 , 914-922, (2008)] |
|
Separation of chitooligosaccharides and the potent effects o...
2009-11-01 [Int. J. Biol. Macromol. 45 , 432-436, (2009)] |
|
Anti-insulin-like (diabetogenic) peptides in pituitary extra...
1984-10-01 [Arch. Biochem. Biophys. 234(1) , 214-229, (1984)] |