Thermodynamics of the interaction of Pd(dmen)(H₂O)₂²⁺ with bio-relevant ligands with reference to the deactivation of metal-based drug by thiol ligands.
Mohamed R Shehata, Mohamed M Shoukry, Sara Ali
文献索引:Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 91 , 383-8, (2012)
全文:HTML全文
摘要
Pd(dmen)Cl(2) complex was synthesized and characterized, where dmen=N,N-dimethylethylenediamine. Stoichiometry and stability constants of the complexes formed between [Pd(dmen)(H(2)O)(2)](2+) and various biologically relevant ligands such as amino acids, peptides and dicarboxylic acids are investigated at 25 °C and at constant 0.1M ionic strength. The concentration distribution diagrams of the various species formed are evaluated. The equilibrium constants for the displacement of coordinated ligands as inosine, glycine or methionine by cysteine are calculated. The results are expected to contribute to the chemistry of tumour therapy.Copyright © 2012 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
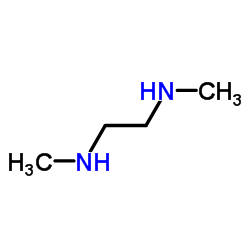 |
N,N'-二甲基乙二胺
CAS:110-70-3 |
C4H12N2 |
|
Redox effects and cytotoxic profiles of MJ25 and auranofin t...
2015-06-30 [Oncotarget 6 , 16488-506, (2015)] |
|
EPR spectra of Cu(2+) doped [Zn(sac)2(dmen)] and [Zn(sac)2(p...
2007-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 68(2) , 394-8, (2007)] |
|
Deprotection of the indole (N(ind))-formyl (For) group on tr...
2009-02-01 [Chem. Pharm. Bull. 57(2) , 211-3, (2009)] |
|
Parallel synthesis of a library of benzoxazoles and benzothi...
2006-03-03 [J. Org. Chem. 71(5) , 1802-8, (2006)] |
|
Transition-metal-free intramolecular N-arylations.
2012-04-06 [Org. Lett. 14(7) , 1892-5, (2012)] |