| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
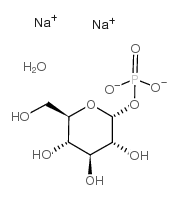 |
A-D-葡萄糖-1-磷酸-二钠盐
CAS:56401-20-8 |
|
 |
尿苷-5′-二磷酸葡萄糖焦磷酸化酶 来源于面包酵母
CAS:9026-22-6 |