Science
1984-04-27
Inhibition of dihydropteridine reductase by novel 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine analogs.
C W Abell, R S Shen, W Gessner, A Brossi
文献索引:Science 224(4647) , 405-7, (1984)
全文:HTML全文
摘要
Hydroxylated derivatives of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a nigrostriatal neurotoxin in humans and primates, noncompetitively inhibited dihydropteridine reductase from human liver and rat striatal synaptosomes in vitro at micromolar concentrations. In contrast, MPTP and its chloro- and norderivatives did not inhibit this enzyme at lower than millimolar concentrations. Dihydropteridine reductase converts dihydrobiopterin to tetrahydrobiopterin, the required cofactor for the hydroxylation of aromatic amino acids during the synthesis of dopamine and serotonin.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
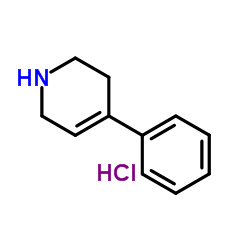 |
4-苯基-1,2,3,6-四氢吡啶盐酸盐
CAS:43064-12-6 |
C11H14ClN |
相关文献:
更多...
|
4-Phenyl-1,2,3,6-tetrahydropyridine, an excellent fragment t...
2005-10-01 [Bioorg. Med. Chem. Lett. 15(19) , 4221-5, (2005)] |
|
Polygalae radix inhibits toxin-induced neuronal death in the...
2011-03-24 [J. Ethnopharmacol. 134(2) , 414-21, (2011)] |
|
Hydrogen in drinking water reduces dopaminergic neuronal los...
2009-01-01 [PLoS ONE 4(9) , e7247, (2009)] |
|
Neuroprotective effects of an herbal medicine, Yi-Gan San on...
2010-09-15 [J. Ethnopharmacol. 131(2) , 433-42, (2010)] |
|
4-phenylpyridine and three other analogues of 1-methyl-4-phe...
1987-03-20 [Neurosci. Lett. 75(1) , 65-70, (1987)] |