| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
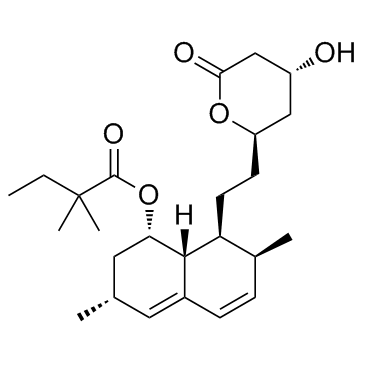 |
辛伐他汀,斯伐他汀
CAS:79902-63-9 |
|
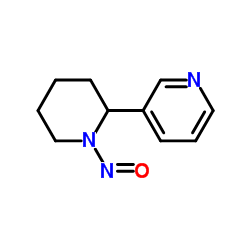 |
N-亚硝基新烟草碱
CAS:37620-20-5 |
|
 |
杀鼠灵
CAS:81-81-2 |
|
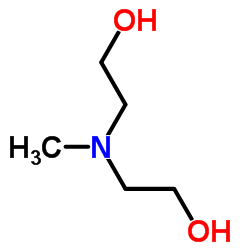 |
N-甲基二乙醇胺
CAS:105-59-9 |